Policy & Regulation
UK Imposes New Restrictions on Methyl Salicylate in Cosmetics

UK Restricts Methyl Salicylate Cosmetics: New Regulations Announced
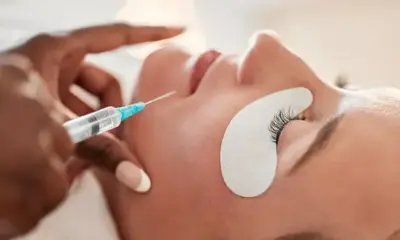
The UK government has made significant updates to its regulations, focusing on the safety of cosmetics. UK Restricts Methyl Salicylate Cosmetics, a compound commonly used in beauty products, has led to new restrictions on its use. This move comes after growing safety concerns, particularly regarding the risks posed to children and the potential for allergic reactions.
New Limits on Methyl Salicylate in Cosmetics
The UK’s Office for Product Safety and Standards (OPSS) recently announced the new rules on methyl salicylate, which is found in a variety of cosmetics. This compound, derived from wintergreen leaves, is popular in products like muscle relief creams, lipsticks, and mouthwashes. However, the OPSS has acknowledged the potential risks of this ingredient, especially if ingested, leading to the imposition of stricter guidelines. UK Restricts Methyl Salicylate Cosmetics to ensure safer use across various personal care items.
What the New Regulations Entail
The updated regulations specify new limits for methyl salicylate in different types of products. For example, rinse-off products like skin and hair care have a concentration limit of 0.06% for adults and children over one year. However, for products intended for children between 0.5 and 1 year, the allowable concentration drops to just 0.02%. This careful regulation aims to protect children, who are more susceptible to allergic reactions.
Additionally, lipsticks and lip balms are allowed a concentration of up to 0.03% for products for children above one year and adults. These limits are set to ensure that consumers can enjoy the benefits of methyl salicylate without facing harmful health consequences.
Key Product Categories Affected
Here are some of the product categories impacted by the new restrictions:
- Rinse-off products (e.g., skin and hair care): 0.06% for adults, 0.02% for children aged 0.5-1 year.
- Lipsticks and lip balms: 0.02% for children 0.5-1 year, 0.03% for older children and adults.
- Toothpaste: Up to 2.5% concentration.
- Mouthwash: 0.1% for children aged 6-10 years, and up to 0.4% for older children and adults.
These new limits offer better protection while maintaining the functionality and benefits of the ingredient in cosmetics.
Why These Changes Matter
The UK’s move to restrict methyl salicylate cosmetics is driven by concerns about health risks, particularly for children. Allergic reactions such as rashes and breathing difficulties have raised alarms in the beauty industry. This revised regulation ensures that consumers, especially younger ones, can safely enjoy their beauty products without unnecessary health risks.
For more news on the latest regulations and trends, explore more articles on our website!