Policy & Regulation
New Safety Rules for Cosmetic Injectables Announced by Australian Regulators
Injectable Treatment Safety Regulations: What You Need to Know
New rules are changing the cosmetic injectables industry. Health authorities now focus on ensuring safety and ethical practices for all practitioners. These injectable treatment safety regulations are necessary as demand for non-invasive cosmetic treatments continues to grow across Australia.
Why New Regulations Matter
The Australian Health Practitioner Regulation Agency (AHPRA) announced fresh guidelines to safeguard patients receiving aesthetic treatments. The new rules apply to nurses and dentists who provide cosmetic injectables. These professionals previously performed procedures without extra education in aesthetic medicine.
Until now, there were gaps in training requirements for non-medical cosmetic providers. AHPRA now insists that all providers meet unified safety standards. These changes reflect concerns about commercial interests taking priority over patient well-being. Therefore, regulations ensure that patient safety comes first.
What the New Guidelines Require
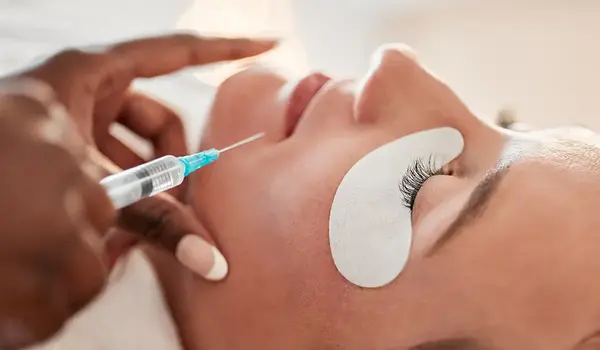
Health practitioners must now undergo additional training before offering procedures like Botox or filler injections. This rule ensures they understand proper technique, risks, and patient aftercare.
AHPRA and the National Boards published guidelines that align with existing medical practitioner standards. These updated rules apply from September 2, 2025, and are part of broader Injectable Treatment Safety Regulations.
To practice cosmetic injectables, nurses must complete 12 months of full-time clinical experience. They must also demonstrate ongoing commitment to ethical practice and patient protection. This includes understanding that the prescriber stays responsible for the patient’s safety throughout the treatment.
Patients should now verify a provider’s credentials on the AHPRA website before undergoing any treatment.
Tighter Advertising Controls
Regulators are also cracking down on how cosmetic treatments are marketed. The new rules restrict promotions that target individuals under 18. Advertisers can no longer direct non-surgical cosmetic campaigns toward minors through social media.
Young people under 18 must also observe a seven-day cooling-off period after their initial consultation. These Injectable Treatment Safety Regulations also stop social media influencers from endorsing cosmetic services. These steps are designed to curb misinformation and reduce impulsive decisions.
According to AHPRA CEO Justin Untersteiner, all advertising must comply with these new standards. Legitimate information must support all ads, especially when promoting higher-risk cosmetic procedures.
Responding to Consumer Concerns
AHPRA’s investigation between September 2022 and March 2025 revealed numerous safety issues. The agency reviewed 360 complaints about non-surgical cosmetic treatments. Over 300 of these cases have since been resolved.
These complaints involved various professions, including dentists, nurses, psychologists, and midwives. In response, authorities launched a cosmetic surgery hotline. This has received over 1500 calls since its creation, highlighting public concern.
The rise of social media marketing prompted extra caution from regulators. Gen Z and Millennials now expect transparency, ethics, and eco-friendly practices from beauty brands and clinics. These expectations shaped the new rules.
Global Focus on Regulation
The push for stricter guidelines isn’t limited to Australia. Other nations are also acting. In South Korea, regulators revised cosmetic standards under the Cosmetics Act. These changes include banning misleading labels and false organic claims.
South Korea also addressed influencer marketing by tightening advertising rules. The country’s food and drug safety agency wants to protect consumers and expand its cosmetics industry globally.
Likewise, England recently banned remote prescriptions for injectables. Patients must now consult prescribers face-to-face before receiving Botox or dermal fillers. These changes reflect a growing international focus on safety.
A New Era for Cosmetic Injectables
The cosmetic industry faces more scrutiny than ever. These changes aim to protect vulnerable consumers and build trust in aesthetic medicine. By enforcing Injectable Treatment Safety Regulations, authorities help ensure ethical practices and long-term industry integrity.
Explore more news on this website to stay informed about health regulations, cosmetic safety, and industry updates.